On the limitations of chemical intuition in drug design
Simple tricks don't always work, but can suggest experiments
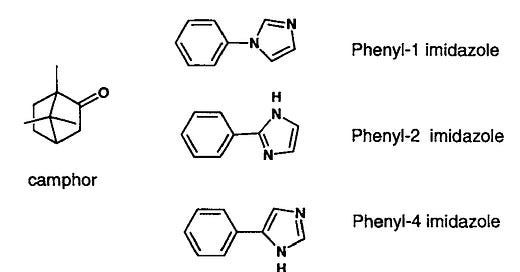
Here's an old but instructive example of why chemical intuition does not always help in drug design. The example is from an article by N. Claude Cohen (Guidebook on Molecular Modeling in Drug Design, 1996, p.1) , a very successful drug designer who designed Aliskiren, the first renin inhibitor for high blood pressure. Cohen gives the example of designing some phenyl imidazole inhibitors for cytochrome P450. It was known that camphor gets hydroxylated by CYP450 and that these inhibitors block its hydroxylation.
A preliminary look at the inhibitors suggests that they are very similar and only differ in the substitution patterns of the phenyl rings coming off the imidazole.
Instinct would lead a modeler or medicinal chemist to do a simple 2D overlay of these compounds as a guide to a common pharmacophore.
But that overlay would not take into account the chemistry of the attachment, since it would end up with nitrogens overlapping with carbons. So we would try a different arrangement in which identical atoms overlap.
This leads to a very different overlay since the two phenyl rings are now offset. The question is: which overlay would we prefer? While it would be difficult to say without further experimental, Occam's razor would suggest that the first one might be better. But as Garland Marshall once said, "Nature does not always shave with Occam's razor."
It turns out that "neither" is the answer here, since crystallography cut that Gordian knot by illuminating a very different answer. Crystal structures of all three analogs bound to the enzyme revealed that the three similar compounds bound in very different orientations: one of them made a hydrogen bond with a Tyr, similar to the OH group in hydroxylated camphor, but the other two had orientations in which the nitrogen atoms chelated with the heme iron in the enzyme.
In the absence of structural and SAR data that clearly would have suggested a different set of behaviors for the three compounds, it would likely have been very hard to predict these very different orientations for what appeared, prime facie, as very similar compounds.
It's an instructive lesson in at least four different aspects of drug design: first, that simple 2D overlays, while sometimes remarkably effective, can also be misleading; second, that crystal structures are invaluable in illuminating unexpected binding modes; third, that modeling in the complete absence of data can be done, but carries low confidence and should be communicated as such; and fourth, that modeling can be used to suggest data that could validate or invalidate a particular model. Caveat emptor, as they say.